Lewis Structures Of Two Hf Molecules Interacting

Generally these are molecules with central atoms from groups 2 and 12 outer atoms that are hydrogen or other atoms that do not form multiple bonds.
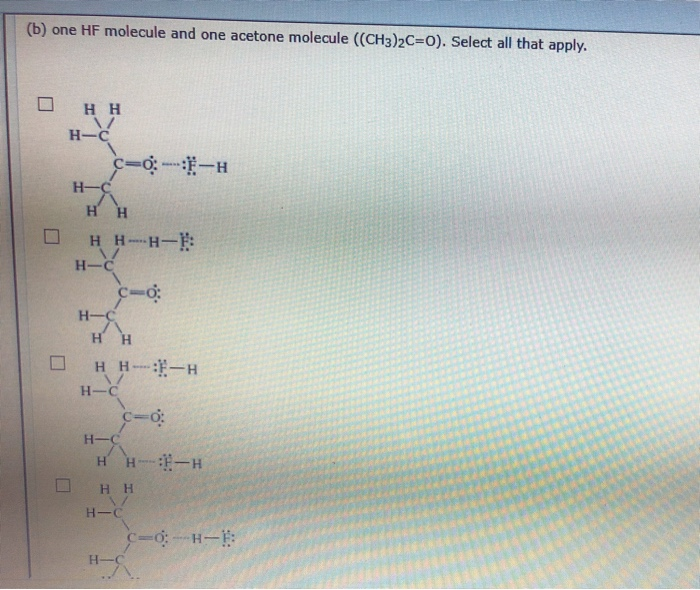
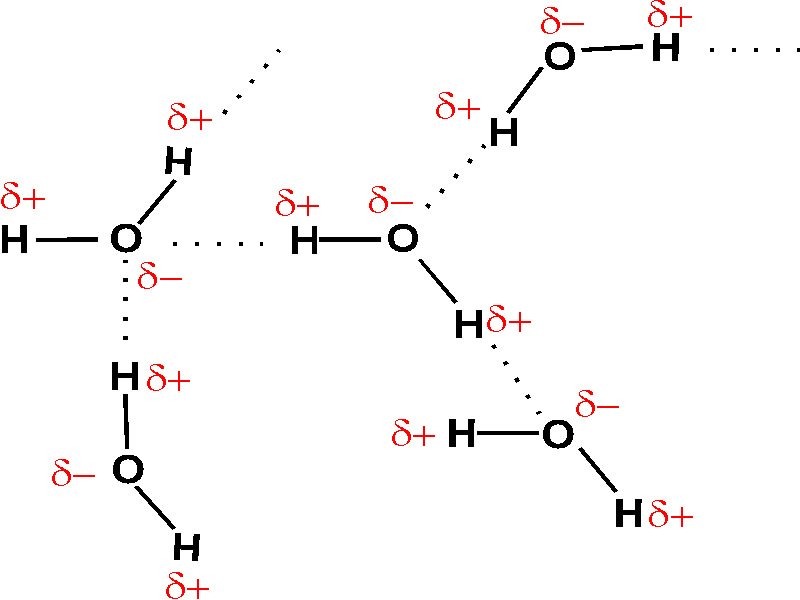
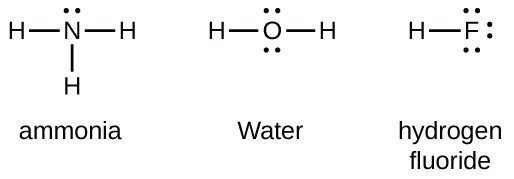
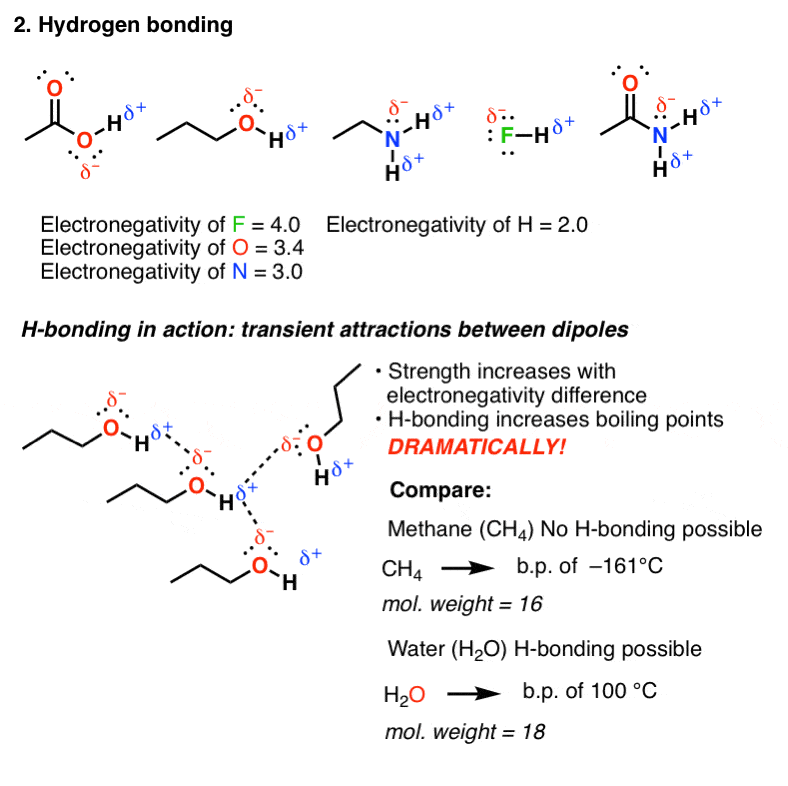
Lewis structures of two hf molecules interacting. Circle the imf that gives rise to the very high boiling point in comparison to other hydrogen halides. Linear structure showing hydrogen bonding between hf molecules and sigma positive and sigma negative charges. The imfs have a lower effect on the boiling point of ammonia than if it were the case of water or hf. A step by step explanation of how to write the hf lewis structure hydrofluoric acid.
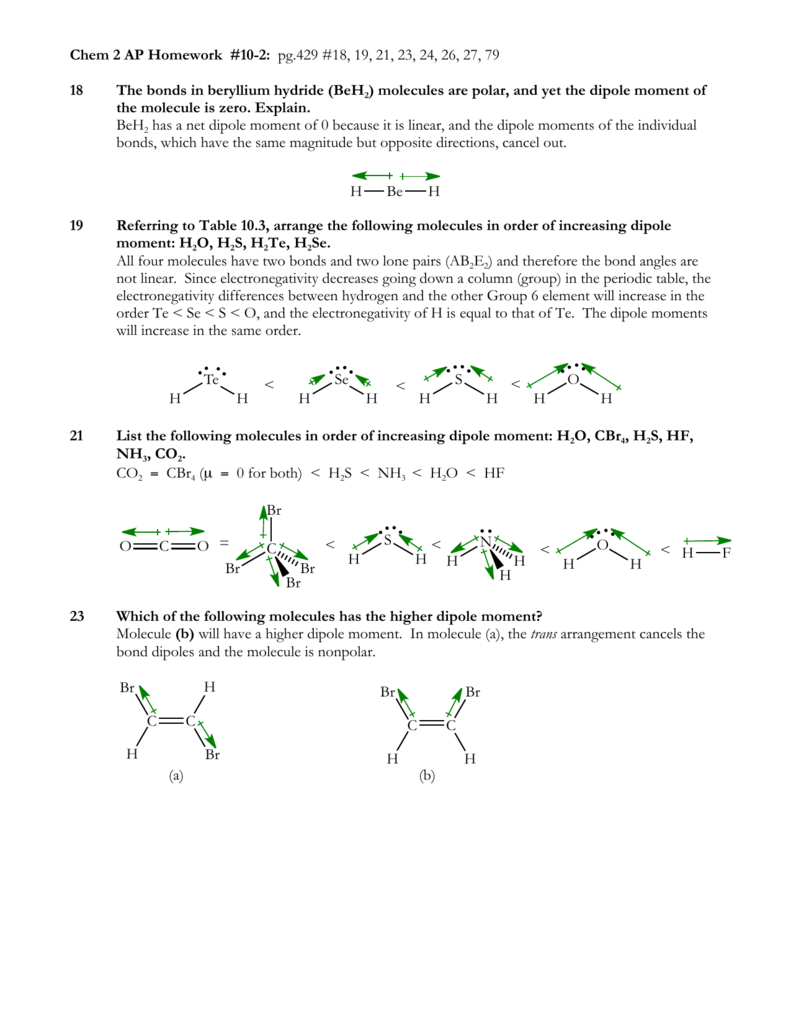
For example in the lewis structures of beryllium dihydride beh 2 and boron trifluoride bf 3 the beryllium and boron atoms each have only four and six electrons respectively. Circle the imf that gives rise to the very high boiling point in comparison to other hydrogen halides expert answer. Ammonia nh3 has the same types of imfs as hf. Draw the lewis structures of two hf molecules interacting in a stabilizing geometry.
We also use lewis symbols to indicate the formation of covalent bonds which are shown in lewis structures drawings that describe the bonding in molecules and polyatomic ions for example when two chlorine atoms form a chlorine molecule they share one pair of electrons.