Hf Shape And Polarity

However to determine if hf is polar we consider the molecular geometry.
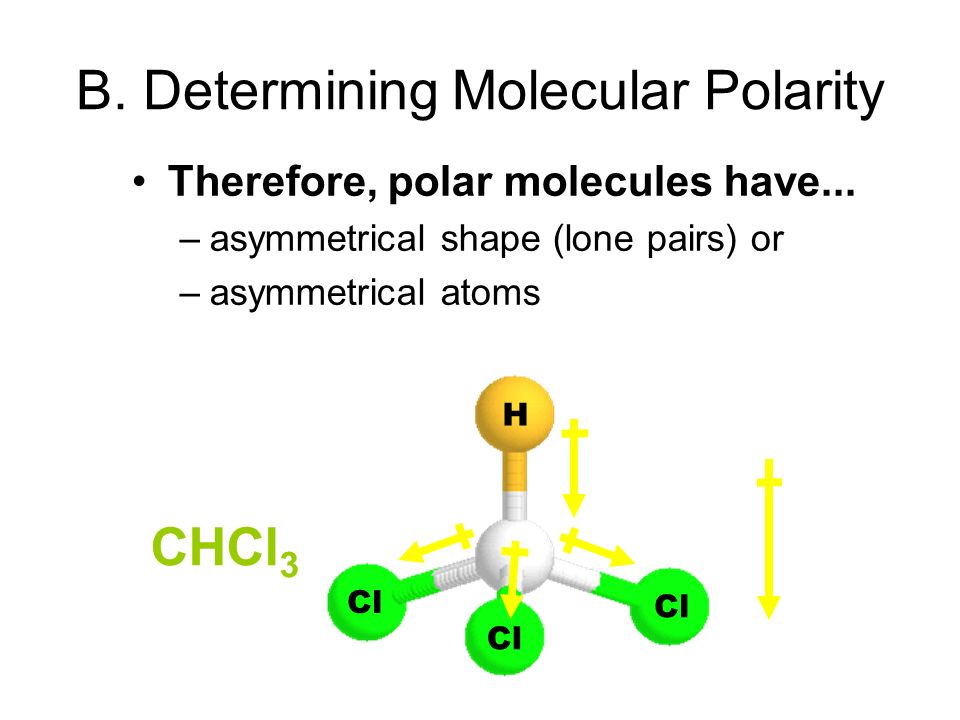
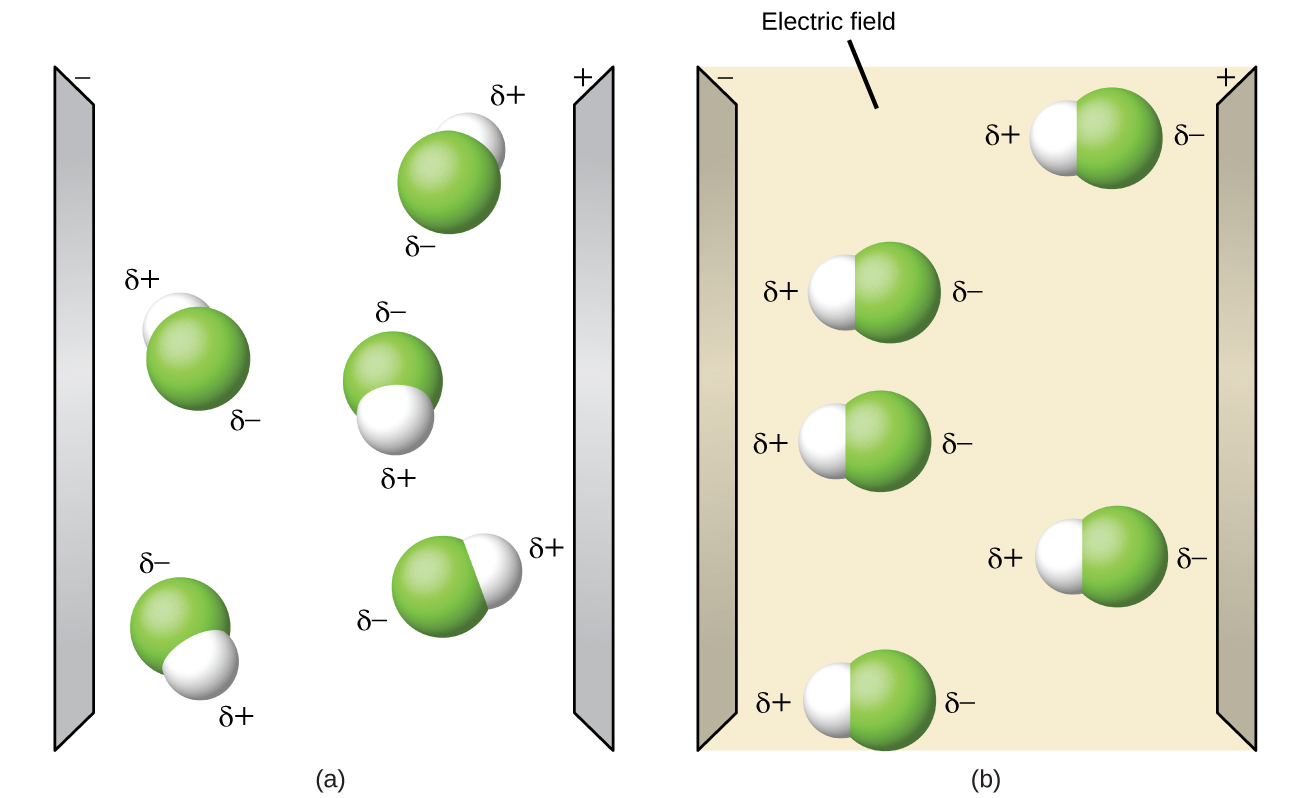
Hf shape and polarity. A diatomic molecule like hf mentioned above has no issue of shape. How does molecular shape affect polarity. A polar molecule is always attracted to the opposite sides of an electric field. The polarization of a molecule greatly depends on the shape of the molecule.
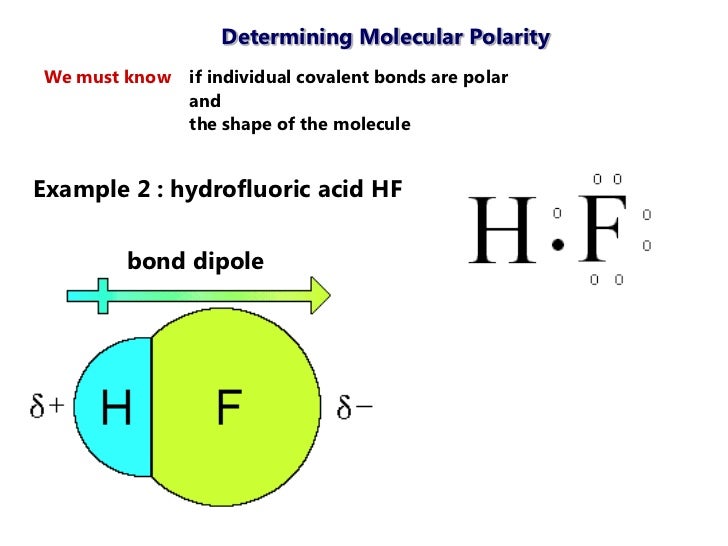
Hydrogen fluoride hf is a compound that is primarily polar. Shown in the figure below. This is due to the high electronegativity of the fluorine that pulls the shared electron pair between h and f more towards its side. In this section polarity of entire molecule will be discussed.
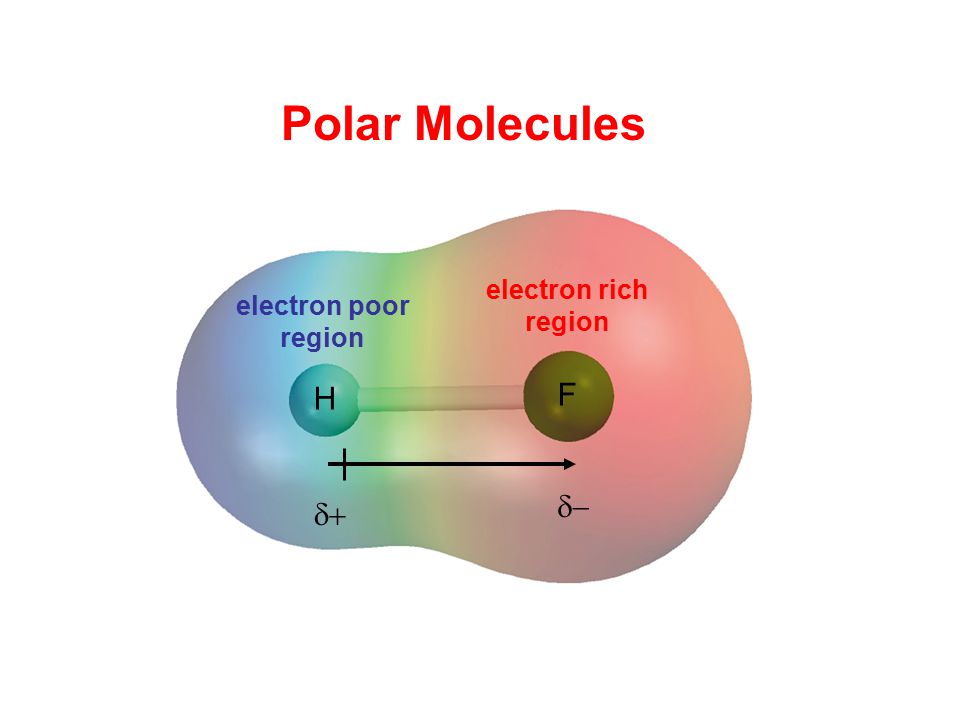
Molecular polarity depends on the individual bond polarity as well as symmetry of the molecule. When a molecule contains more than one bond the geometry must be taken into account. A polar molecule results from an. This leads to the development of a partial negative charge on the f atom and a partial positive charge on the h atom leading to the generation of a dipole and hence polarity.
The polar covalent bond hf. Note that molecular shape influence polarity so molecules with the same elements but a different shape and vice versa won t. However molecular structure is actually three dimensional and it is important to be able to describe molecular bonds in terms of their distances angles and relative arrangements in space figure 7 14 a bond angle is the angle between any two bonds that include a common atom usually measured in degrees. The resulting hydrogen atom carries a partial positive charge.
This results in a net dipole moment in a molecule. If you look at the lewis structure for hf it appears to be a symmetrical molecule. Thus far we have used two dimensional lewis structures to represent molecules. The highly negative f in the hf molecule gets a slight negative charge while the h atom becomes slightly positive.
The more electronegative 4 0 2 1 fluorine pulls the electrons in the bond closer to it forming a partial negative charge. For hf there is a larger dipole moment because there is a larger difference in electronegativity.


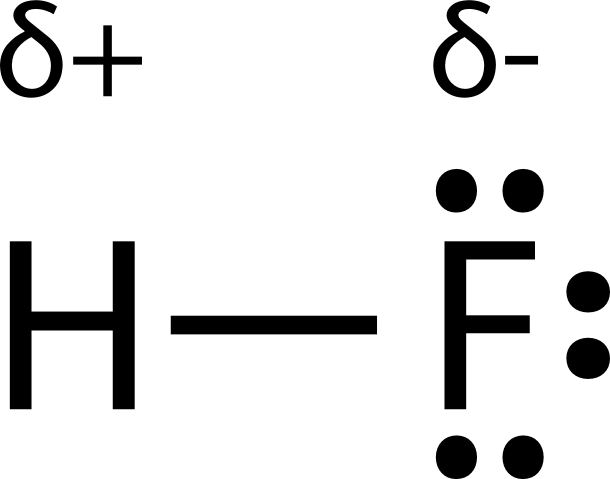








.PNG)

.PNG)