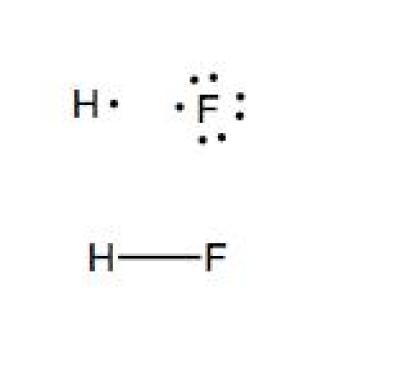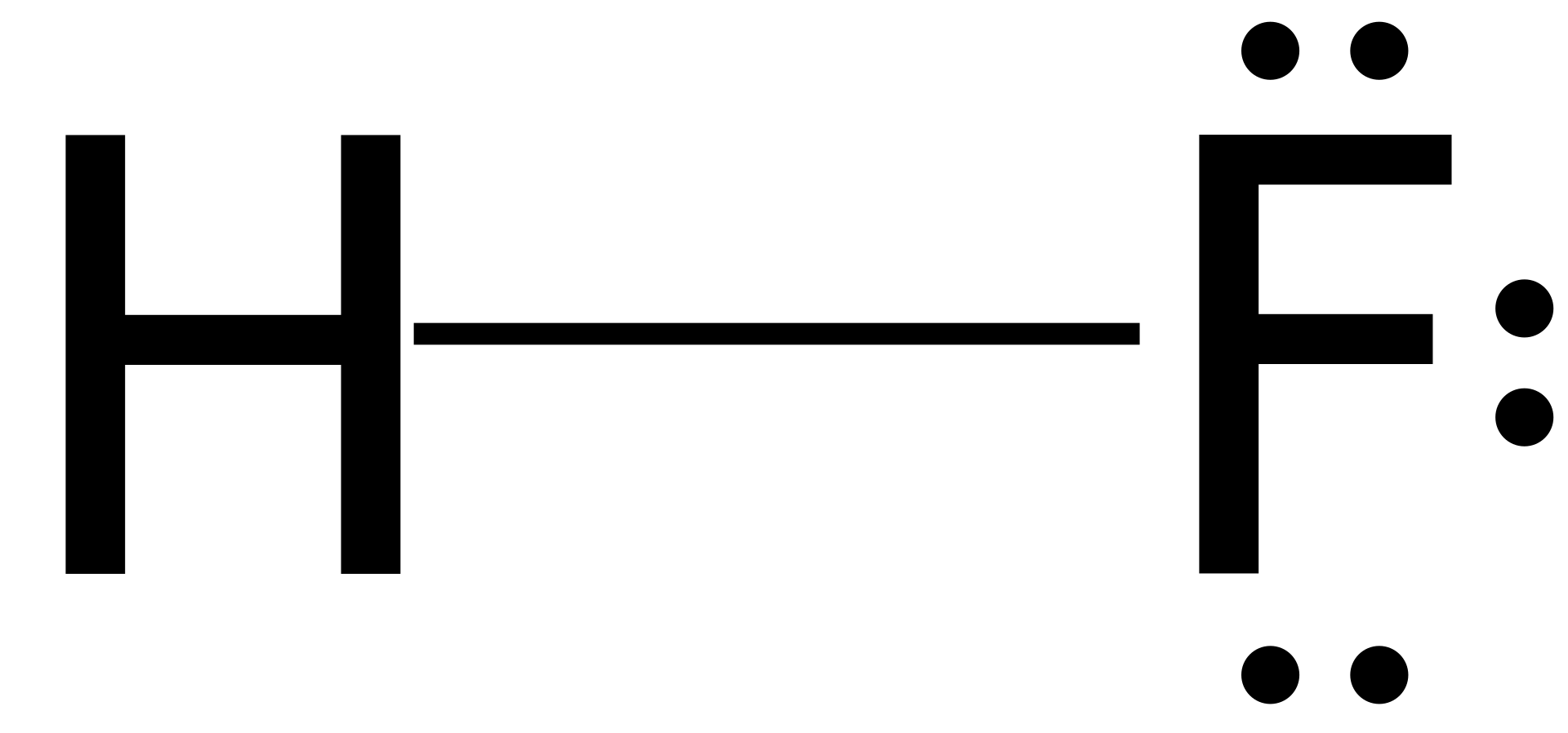Hf Dot Structure

The lewis dot structure.
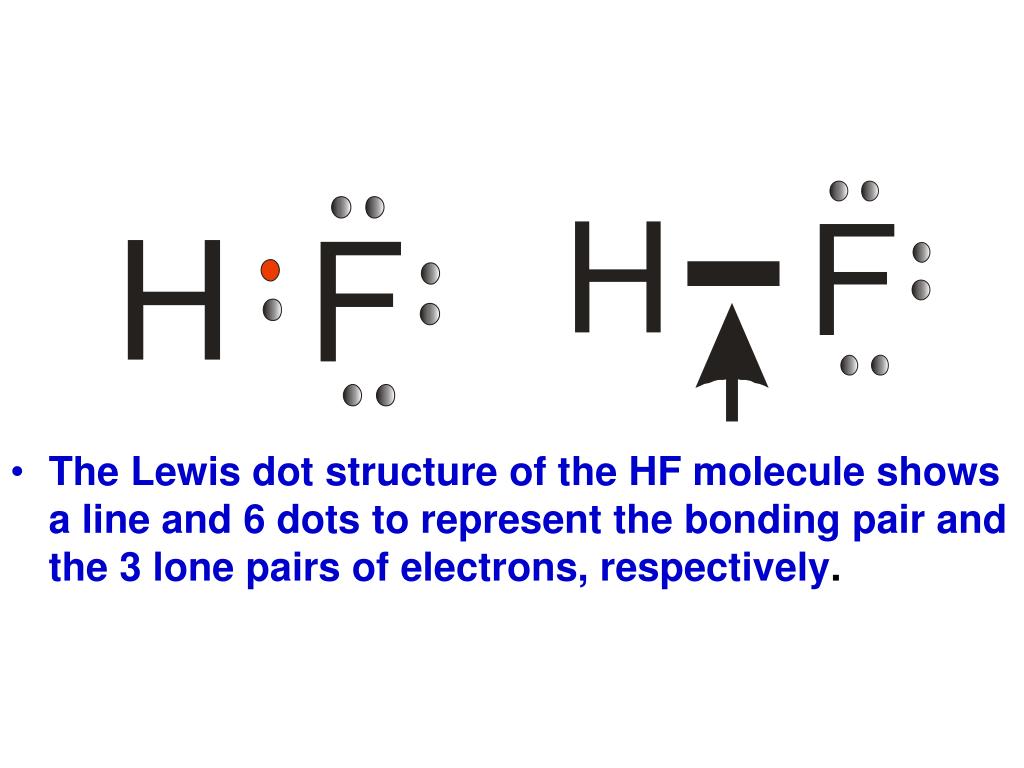
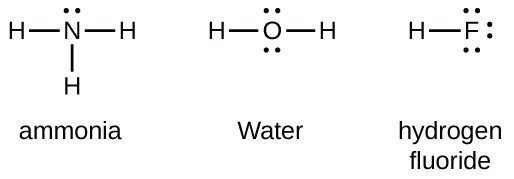
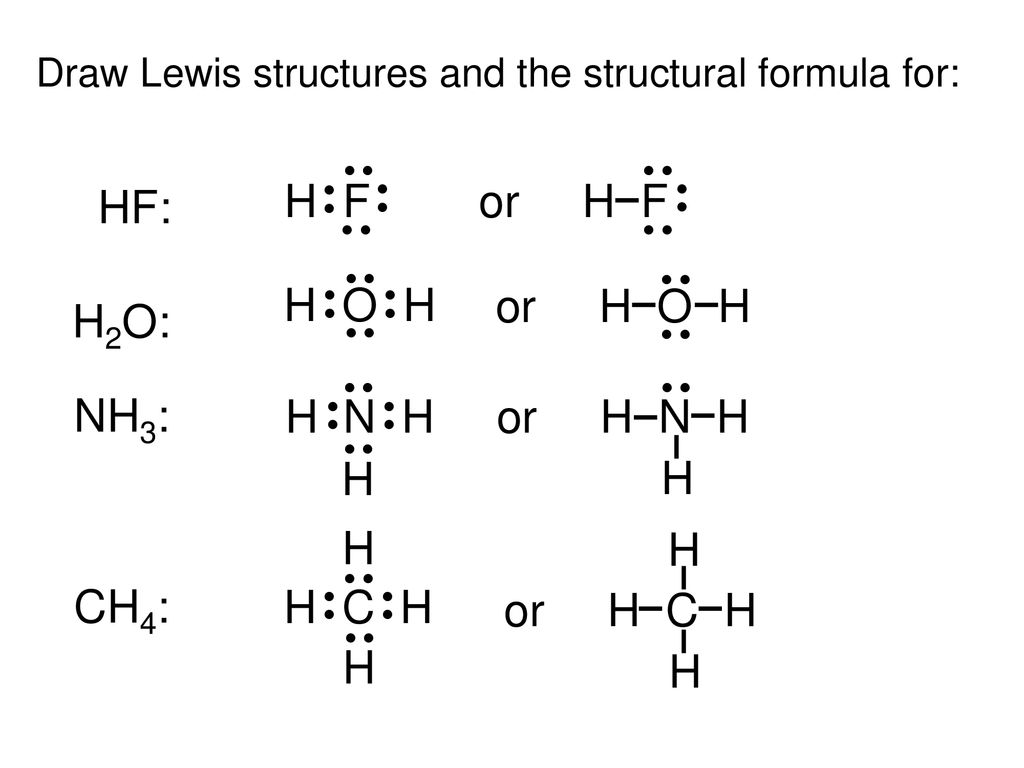
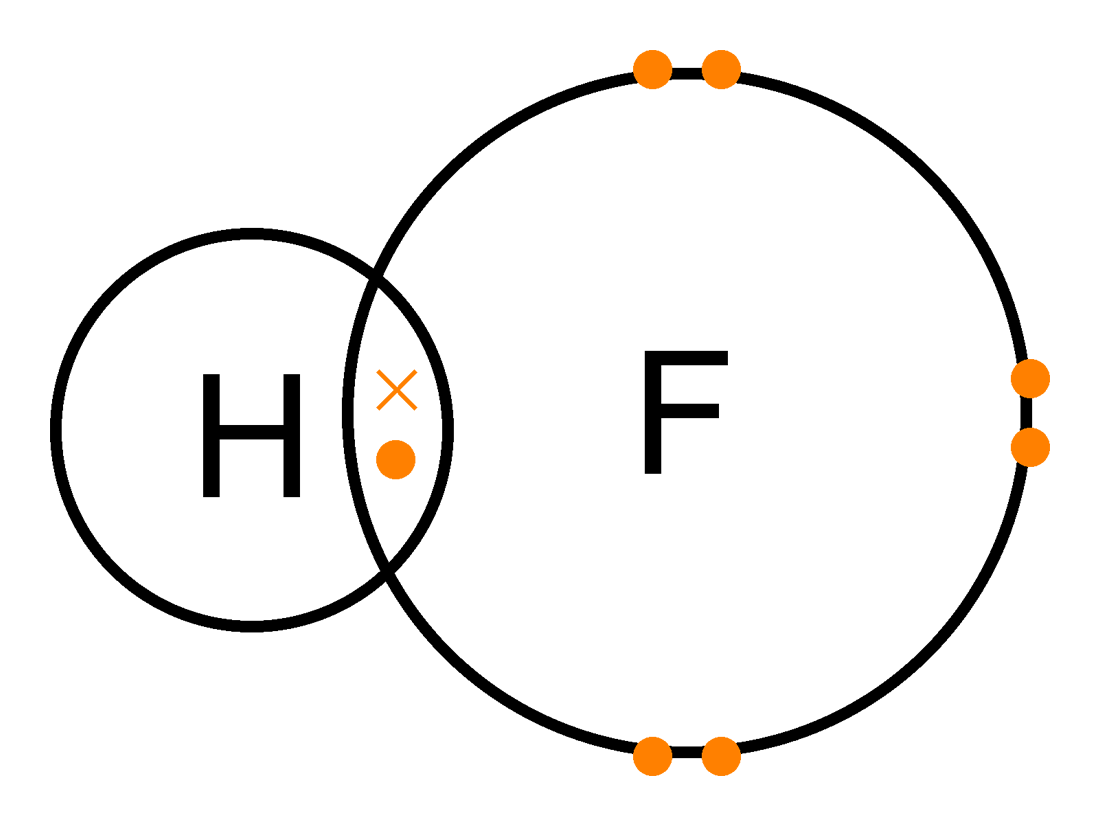
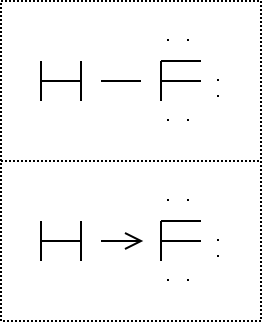
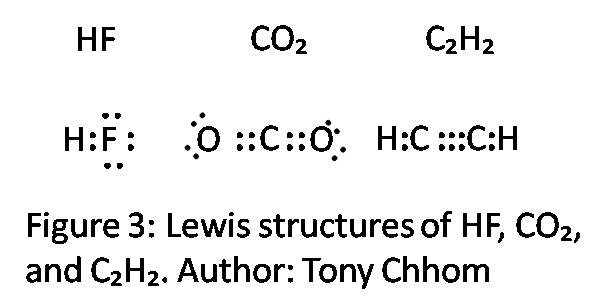
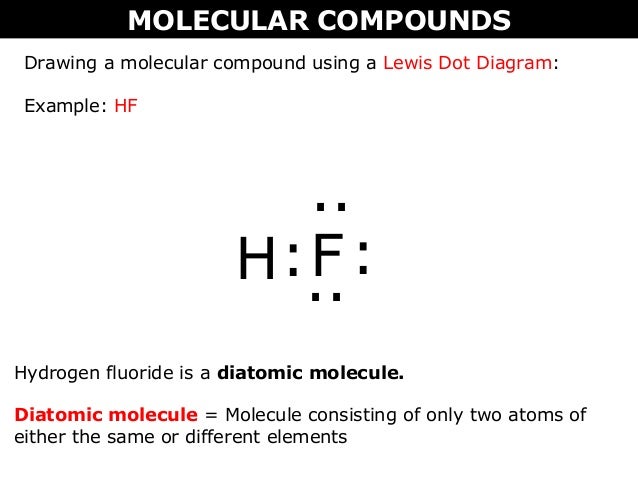
Hf dot structure. The exponets are 6 2. This huge difference in the hf bond leads to a fervent polarisation of the bond which is also evident in the electrostatic potential map. The lewis dot structure is a diagram showing the electrons. Wayne breslyn 38 890 views.
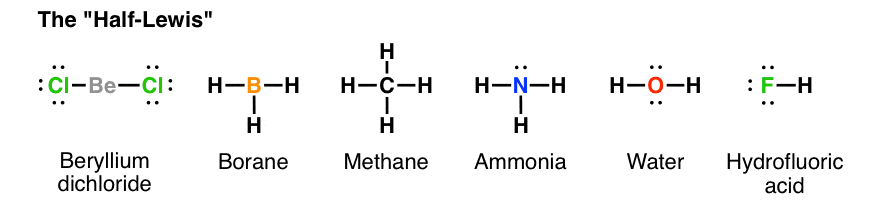
Hf is very similar to hf and hcl. Find more chemistry widgets in wolfram alpha. Hydrogen has 1 valence electron and fluorine in group 7 with f and cl has 7 valence electrons. Get the free lewis structure widget for your website blog wordpress blogger or igoogle.
70 more lewis dot structures. Write the atomic symbols for the atoms involved so as to show which atoms are connected to which. With the lewis structure for hf remember that hydrogen only needs 2 valence electrons to have a full outer shell. This is due to hydrogen bonding that occurs between molecules.
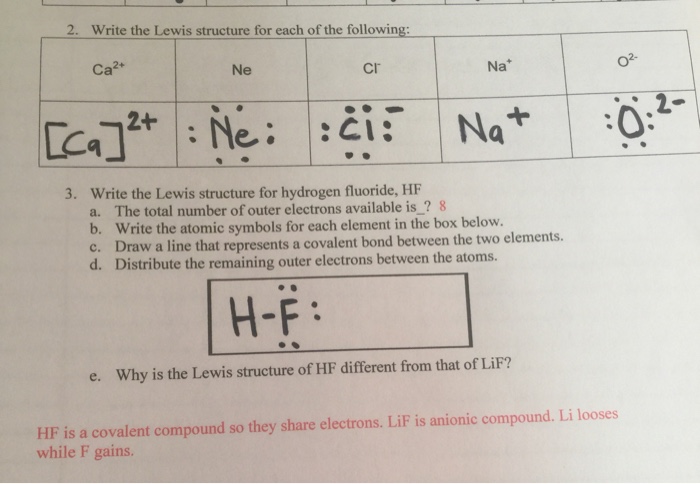
A step by step explanation of how to write the hf lewis structure hydrofluoric acid. Bonding molecular structure page 44 lewis dot structure of hydrogen fluoride. Draw a single bond between each pair of bonded atoms. First we count the valence electrons for hf using the periodic table.
Hafnium as an example the highest number in the electron configuration would be 5. Hf lewis structure how to draw the dot structure for hf duration. Hydrofluoric acid hf or fh cid 14917 structure chemical names physical and chemical properties classification patents literature biological activities. Unlike the other hydrogen halides hf is lighter than air and it is particularly penetrating which can damage the lungs.
Kip thorne at cardiff university duration. 931 55 blue orange. Your suppose to add them together. The warped side of the universe.
Drawing lewis structures sum the valence electrons from all atoms in the species. That would be 8.