2 Hf Lewis Structure
For the f lewis structure use the periodic table to find the total number of valence e.
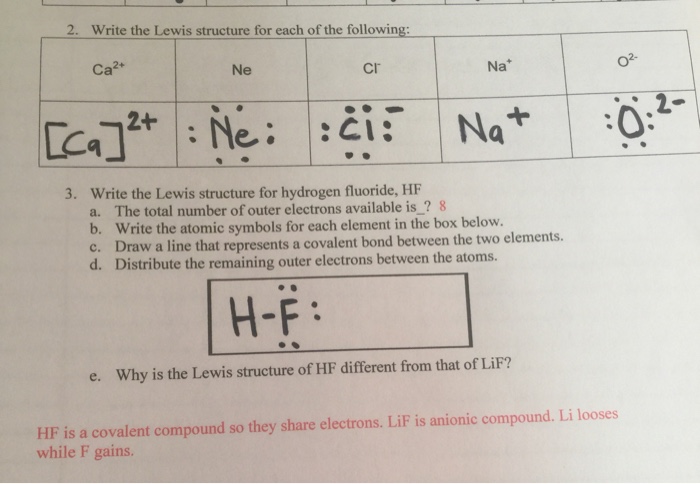
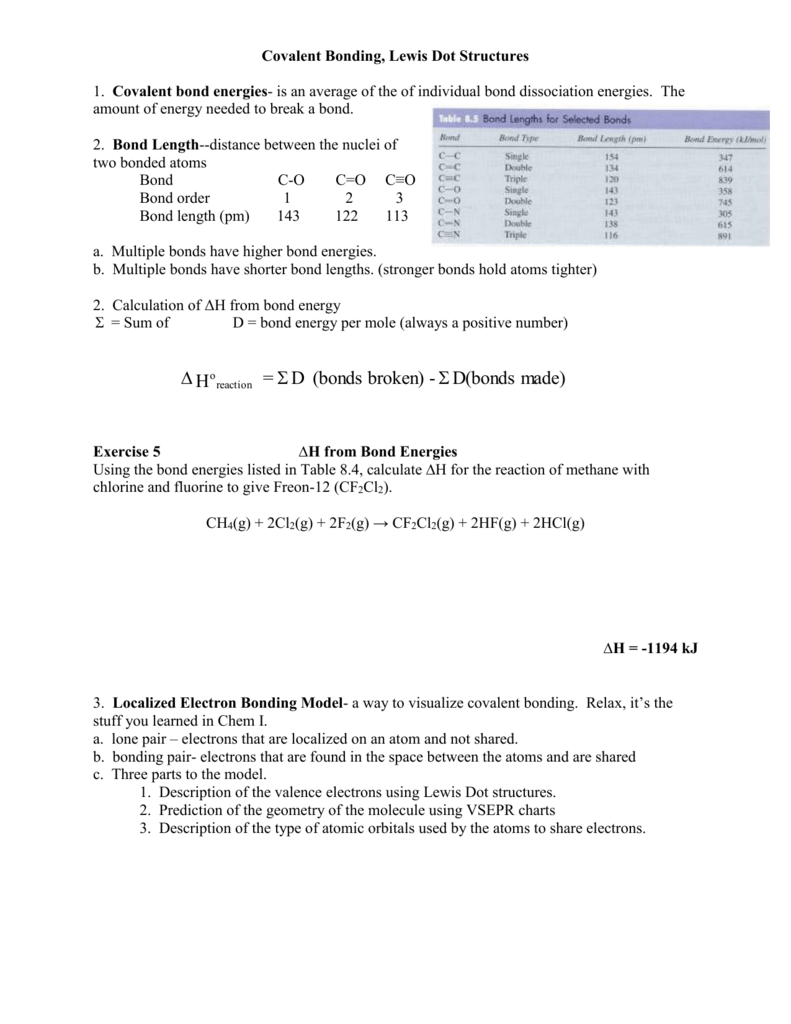
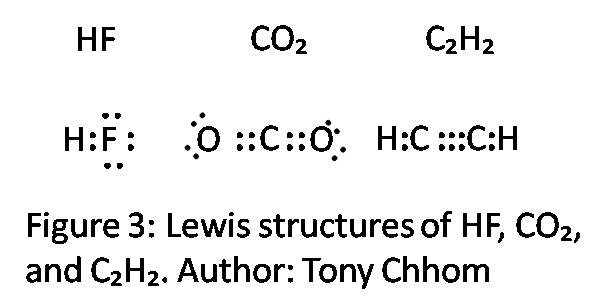
2 hf lewis structure. Geometry maximizes separation. Include non bonding pairs but not multiple bonds. With the lewis structure for hf remember that hydrogen only needs 2 valence electrons to have a full outer shell. Be sure that you don t use more than the 8 valence electrons available.
How to draw the lewis structure of n2 with explanation. See the big list of lewis structures. Find more chemistry widgets in wolfram alpha. Moreover by sharing a bonding pair with oxygen each hydrogen atom now has a full valence shell of two electrons.
Hydrogen has 1 valence electron and fluorine in group 7 with f and cl has 7 valence electrons. Get the free lewis structure widget for your website blog wordpress blogger or igoogle. A step by step explanation of how to write the lewis dot structure for hi hydroiodic acid. Liquid hf also consists of chains of hf molecules but the chains are shorter consisting on average of only five or six molecules.
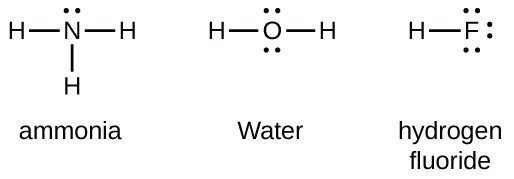
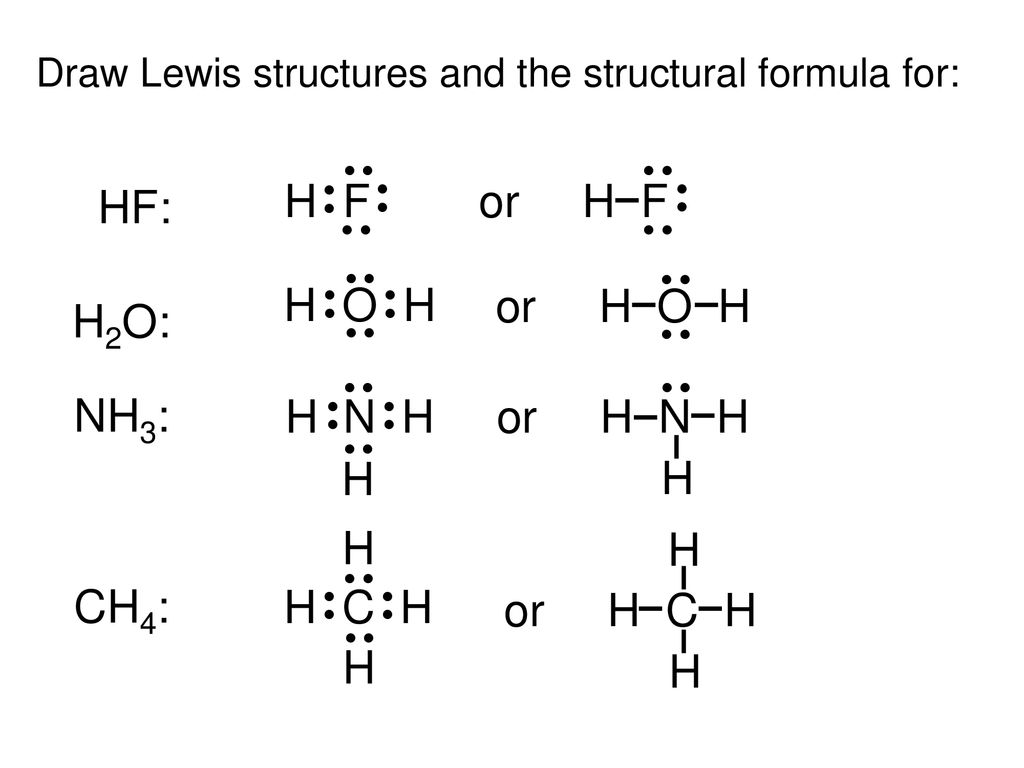
A step by step explanation of how to write the hf lewis structure hydrofluoric acid. Lewis structure if needed. Solid hf consists of zig zag chains of hf molecules. The reason is formal charge and the fact that.
Sulfur make six bonds in this lewis structure. Two of the oxygens are single bonded and two are double bonded. The hf molecules with a short h f bond of 95 pm are linked to neighboring molecules by intermolecular h f distances of 155 pm. With two bonding pairs and two lone pairs the oxygen atom has now completed its octet.
First we count the valence electrons for hf using the periodic table. E pairs geometry example 2 linear hf 2 3 equilateral triangular bf 3 4 tetrahedral td cf 4 5 trigonal bipyramidal tbp pf 5 6 octahedral oh sf 6 7 pentagonal bipyramidal if 7 8 square antiprismatic taf 8 3 6 drawing oh and td. A step by step explanation of how to draw the f lewis dot structure.